Standard enthalpy of reaction
The standard enthalpy of reaction (denoted ΔrH⊖) is the enthalpy change that occurs in a system when one mole of matter is transformed by a chemical reaction under standard conditions.
For a generic chemical reaction
- −vA A + −vB B + ... → vP P + vQ Q ...
the standard enthalpy of reaction ΔrH⊖ is related to the standard enthalpy of formation ΔfHo of the reactants and products by the following equation:
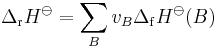
In this equation, vB is the stoichiometric coefficient of entity B.
A similar enthalpy change is the standard enthalpy of formation, which has been determined for a vast number of substances. The enthalpy change of any reaction under any conditions can be computed, given the standard enthalpy of formation of the reactants and products.